
A condom and use manual from 1813 (Wikipedia)
When it comes to reversible birth control, there are a lot of options out there for women: pills, rings, intra uterine devices, patches, implants. Men, on the other hand, are still limited to the same two contraceptive techniques they’ve been using for centuries: condoms and withdrawal. The question is, why?
“Condoms and coitus interruptus are the only means of reversible birth control in men — and will remain so because the problem is not scientific, but economic,” Carl Djerassi wrote in WIRED in 2013.
Djerassi was a chemist, renowned for synthesizing noresthisterone in the 1950s, an essential component of the first oral contraceptive. His involvement earned him the title, shared with Dr. Gregory Goodwin Pincus, “Father of The Pill.” According to Djerassi, the research path to longer-term, reversible, male birth control was obvious.
“Scientifically, we know how to create a ‘male Pill,’” Djerassi asserted.
But Djerassi also thought that he would never get to see it, because the economics of pharmaceutical development would inevitably get in the way. Some of the more promising scientific discoveries about male contraception are decades old, but were never developed into public products. Because of a combination of legal, social, and biological factors, male contraceptive technology hasn’t been able to attract the necessary research dollars.
Djerassi passed away in January, 2015. But one of his former students, Elaine Lissner, is doggedly trying to solve the scientific and economic problems facing male contraceptive technology. Lissner is director of the Parsemus Foundation, which is currently developing Vasalgel — a non-hormonal, reversible form of male birth control. The technology is simple: an injectable gel clogs the vas deferens and blocks the flow of sperm to the urethra. It is reversed by injecting a solvent to break the clog down.
Other male contraception products are on a “five to ten year” timeline, but according to Lissner, “five to ten years” may as well mean “never.” Lissner is trying to get Vasalgel to market in the next three years. She has her work cut out for her.
***

Elaine Lissner
The Guardian has described Elaine Lissner as, “a fast-talking woman in her 40s.” While apt, this description misses some of her key aspects: Lissner is tenacious, but she’s also warm and funny. And, unsurprisingly, she’s very geeky about contraception. She has been working on advancing male contraception research since 1986, her freshman year of college at Stanford University.
“I was procrastinating in the dorm library,” Lissner says, “and I picked up a book that had a chapter about a Swiss doctor who discovered a method of male contraception in the 1930s.”
She had stumbled upon the work of Dr. Marthe Voegeli. Voegeli had a practice in India from 1930 to 1950. She was initially working with a set of 9 volunteers, and did her best to track their sperm counts and their partners’ rates of conception over 20 years. Voegeli discovered a method that involved soaking the testes in a hot bath once a day for three weeks. 45-minute-soaks in water 116˚ F (46.7˚ C) resulted in 6 months of infertility. Participants who discontinued use of the method went on to successfully have children, with no apparent complications.
To Lissner, the maddening thing about the method was that even though it was eventually used by hundreds of volunteers in India, it was never developed and implemented into an accepted clinical procedure. “Various people did tests and discovered that it worked, and then it never went anywhere,” Lissner says, frustrated.
She later enrolled in a small seminar taught by Carl Djerassi, who was already famous for his role in developing the first oral contraceptive. For the class, she wrote a report on male contraception. Her research turned up eight, non-hormonal methods that were known to work, but not in use.
At the same time, some of Lissner’s female friends were having a hard time with the less desirable side effects of hormonal birth control, episodes she describes as “horror stories.” “It seemed crazy,” she says, “that these better options weren’t being developed.”
The Swiss doctor, Voegeli, had spent the last decades of her life trying to spread knowledge of her so-called “wet heat” method, and to promote further research, to little avail. Lissner started thinking about picking up where Voegeli had left off.
Even then, Djerassi was a skeptic about the viability of male contraception. He said that male contraception would never be developed. As part of her research, Lissner found that, within the health care and pharmaceutical industry, it was believed that there was “no public demand” for new male contraceptives.
Lissner’s class paper turned into a white paper, then a series of short pieces in women’s and men’s magazines. First, she wrote a long article in the now-defunct feminist magazine WomenWise, then shorter pieces in Ms. and Changing Men in 1992. In 1994, her research was published as a chapter in a scholarly book, Issues in Reproductive Technology: An Anthology.
When her article ran in Ms., Lissner received over 200 hand-written letters in response, from both women and men, most of them interested in knowing more about male contraception. The letters from men were particularly notable, considering the article had run in a women’s magazine. “That’s when I realized there really was a demand for this,” Lissner says.
Women were interested in male contraception, men were interested in male contraception. Why weren’t drug companies interested? And why are they not, still?
A Modern History of Contraception
If there’s a market for a product, why wouldn’t an industry step in to exploit the demand?
It was only recently that human contraception became an accepted area of research in Western science. The demand for a product that would better-facilitate sex without the threat of pregnancy existed, but for many years it was considered immoral and obscene. Thus, it was a taboo to attempt to satisfy it.
The effects of the first oral contraceptive pill were so controversial that it was actually, in a way, developed in secret. It was first approved by the FDA in 1957 as a treatment for menstrual disorders — the label included a warning that it would also, as a side effect, prevent ovulation, thus resulting in temporary infertility. By 1959, over half a million women in America mysteriously claimed to have developed severe “menstrual disorders,” and were taking the drug.
It took a while for people to come around to the Pill, and once they had, it was assumed, for a long time, that men would not be interested in having their own version. Contraception was seen, Lissner thinks erroneously, as a “women’s issue.” Yes, the ability to avoid unwanted pregnancy has huge consequences for a woman’s health. But men, for the most part, don’t want unwanted pregnancy either.
“There definitely was resistance,” Lissner says, “and I think it took a lot of years of advocacy to get people to realize that men actually are interested.”
“It shouldn’t be surprising, though. Between condoms and vasectomy, men are already covering about a quarter of the country’s birth control. If you add withdrawal, that’s even more,” Lissner adds. But she says, chuckling, she doesn’t know if she counts withdrawal as a strictly-male form of birth control: “The man needs to do it, and it the woman needs to not say, ‘That feels so good don’t stop!’”
The point is that men use the techniques available to them in great numbers, despite the limitations of those methods. Why should anybody think they wouldn’t be interested in another option? “Maybe generations ago you could say men were not interested, but that’s not a thing today,” Lissner says, “and certainly not in developed countries.”
Nowadays, male contraceptive research is being held back on the supply side, not the demand side. According to Lissner, there is comparatively little commercial incentive to develop a new contraception product at all.
First of all, the market isn’t big enough, by pharmaceutical and healthcare standards: $4 billion nationwide. Contraceptive products take a particularly long time to develop and test, which increases the risk of an investment. “$4 billion may sound like a lot,” says Gary Gamerman, a former consultant to Lissner’s foundation and vice president of Afaxys, Inc., “but when you divide it out among a lot of different products, and take the time and effort to develop and test a completely new product, or just an improvement, you could easily spend $20-$50 million. To launch it you’re going to spend another $30-40 million in marketing. And the whole thing will take 5 years at the very minimum.”

Contraceptive ring (byckttor)
Add to that another complication: Existing contraceptive companies already, for the most part, have that market cornered. Most people who would be interested in a new form of birth control are already using another form of birth control. Any new product pharma companies could introduce would be likely to cannibalize their existing products. After all, one reason for a man to want to use a reversible contraceptive is so his partner can stop taking the pill.
Also, both old and new companies are deterred from investing in developing new contraception techniques because the liability is much higher than in other areas of research.
“Why would you want to get into contraception? In contraception, you’re treating healthy young people for a long time,” Lissner says.
Although any kind of medical product will have the risk of side effects, companies have the option of developing treatments for sick people, where undesirable side effects are offset by curing or treating something worse. Why would you invest in contraception research when you could develop treatments for cancer instead? Lissner asks. As she points out, “With a cancer treatment you can practically kill people with side effects, and still charge them an arm and a leg.”
With female contraception, the less desirable side effects are offset by the health complications that can arise from unwanted pregnancy. Not so with male contraception products. It becomes even more difficult, ethically, to justify negative side effects. According to Aeon, the last major study of hormonal male contraception was sponsored by the WHO and CONRAD, from 2010-2011. It ended prematurely when the advisory board noticed a “higher than expected rate of depression, mood changes and increased sexual desire in the study volunteers.”
From MS. to Penthouse

After the 1989 earthquake, the undergraduate Lissner dropped out of school and started a non-profit: the Male Contraception Information Project. She tried to use it as a platform to raise money for male contraception research and outreach. But in the middle of the HIV/AIDS epidemic, and with the development of mifepristone (then known as RU-486) as an emergency contraceptive, there were a lot of other battles being waged in sexual health. Male contraception got crowded out.
“The Male Contraception Information Project was before its time,” Lissner says. “Eventually I was like, ‘OK, I get the message,’ and I went back to school to finish my undergraduate degree.”
Lissner graduated with her degree in psychology in 1994. But she entered a relatively tough job market, especially in the San Francisco Bay Area, which was still recovering from the damages of the 1989 earthquake. Not knowing what else to do, she followed a passion for Italian and moved to Italy. She lived there a few years, teaching English.
Lissner says, she still felt rudderless when she came back to the United States. “Then in 2001,” she says, “a journalist from Penthouse contacted me wanting to know what was going on in male contraception.”
The journalist had found Lissner through her reports published nearly a decade before, and wanted to do a follow-up piece.
“I called a friend of mine and asked, ‘Should I be involved in this?’” Lissner says. She had misgivings about cooperating with a pornographic men’s magazine. Her friend responded, “Well it is your target market.”
That interview with Penthouse changed the course of Lissner’s life. She called up her old contacts and started investigating male contraception again. She found that, though a lot of the same economic obstacles to developing new products were still in place, great strides had been made in the non-profit and academic sectors.
A few years later, when Lissner came into some cash, she wasted no time in funding research. “That money came from the national housing boom,” she says. “On my father’s side of the family there’s a construction company, building suburban sprawl. I read The Economist, and in 2002 they were already saying there was a housing bubble.”
“I told myself that once I reached a point where I could support myself with savings, then everything else would go into a foundation,” Lissner says. This is how she would make the move from advocacy to action. “I was able to found the Parsemus Foundation at the end of 2005. It felt pretty great to be able to finally be doing something, instead of saying, ‘Somebody ought to do it!’”
One of the first things Parsemus did was test ultrasound contraception. Actually a more-practical variation on Voegeli’s heat method, ultrasound contraception uses ultrasound energy to heat testicles, instead of a hot bath. “It was published in the 70s and never pursued after that,” Lissner says. “Until we dug into it. We sponsored a study in rats, and a study in monkeys, and we just had a study in dogs. Unfortunately, it didn’t work out so well for humans, with their bigger testes. But it turns out to work great for dogs and rats.”
“It was disappointing, but now we know,” Lissner says. “It wasn’t rocket science, it just needed to be done.”
The Non-Profit Backbone of the Contraceptive Industry
Vasalgel
This describes a lot of contraceptive research: not particularly complicated, it just needs to be done.
But given the economic deterrents, even female contraception technology has been neglected by the commercial sector. Most “new” products brought to market are cosmetic reworkings of existing ones. In the last few decades, most of the truly new contraceptive technology was incubated outside the commercial sector and then purchased and brought to market. This includes the Mirena IUD, the most popular hormonal IUD on the market, the new Liletta IUD, and the ParaGard copper IUD. Products like Essure — a tubal ligation alternative — were developed when a small medical devices company was trying to make something else, and then bought by a larger company.
“The core economics of this industry,” Sabrina Martucci Johnson says, “is for large companies to buy mostly-finished products and bring them to market. They grow their portfolios by acquisition, not through their own research.”
Martucci Johnson is the current CFO/COO of Calibr – California Institute for Biomedical Research, and recently founded a startup called DARÉ Bioscience. DARÉ’s mission is to help catalyze technological development in the women’s health and contraceptive sectors by connecting the groups developing the technology — mostly non-profits and academic organizations — to more traditional investment dollars.
The point is that foundation, nonprofit, or government efforts have become essential to developing new contraceptive technology. The Parsemus Foundation is especially important because it is occupying a niche that’s even neglected by most contraception-focused non-profits: male contraception. The Gates Foundation and US Agency for International Development fund contraceptive research. Big groups like this tend to focus on emerging markets. The presiding wisdom is that in these more traditional societies, birth control is still very much a “women’s issue,” and men are less receptive to anything that might limit their virility.
“These agencies have funded wonderful advancements in new products and options for women,” Martucci Johnson says. “We just haven’t seen that for men. That can’t be their focus because of the cultural dynamics in the places they’re working. In a lot of these countries, the more children a man has, the more powerful he is perceived as.”
Parsemus is doing its best to catch male contraception as it falls through the cracks. In 2010, the foundation bought the rights outside India to RISUG: Reversible Inhibition of Sperm Under Guidance. RISUG is a male contraception product that was developed at the Indian Institute of Technology, and would be the model for Vasalgel.
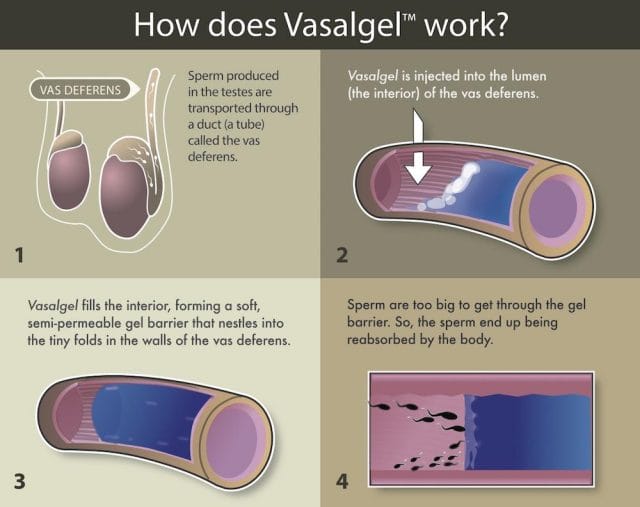
Compared to hormonal birth control, the technology behind RISUG and Vasalgel is simple: the vas deferens is extracted with forceps, as in a vasectomy, and then injected with a gel. When the patient wants his fertility back, they inject the vas deferens with a solvent, and the gel goes away.
RISUG’s gel is thought not to rely on blocking the vas deferens entirely, but also destroy or disable the sperm as they pass through. Vasalgel’s gel is made up of a different, more “shelf stable” formula. Its mechanism is simpler: It forms a semi-permeable plug to block off the vas deferens. The plug seems to allow fluid through, but filters out the sperm. It is a reversible vasectomy without the cutting.
“Part of what’s held male contraception up for so long is a narrowness of thinking,” Lissner complains. She says that for a long time people assumed that reversible male contraception would be hormonal, like the female pill. It wasn’t until the cancellation of the WHO/CONRAD study that non-hormonal approaches started being taken seriously, though they might seem obvious now. “You’ve got this narrow tube that all the sperm have to go through. Why not attack there instead of pumping hormones through the whole body?”
***
RISUG made it all the way to advanced clinical human trials in India, but Vasalgel is still in development for human use. The team is currently working through various species. Vasalgel has proven both durable and reversible for rabbits, and durable for baboons, although baboons have a “very delicate!” vas deferens and reversibility has proven more challenging. They’ve recently started reversibility trials in dogs, which have a “surprisingly” similar vas deferens to humans.
Human trials are expected to start next year, in 2016. If everything goes well, the product could hit the market in 2018.
If that happens, it will likely earn Elaine Lissner the title, “Mother of Vasalgel,” in her teacher’s legacy. And it will probably be the salvation of many would-be fathers, who will be glad to have another option. As a man once wrote to Lissner, “Condoms are a nice method. However, I have a 3-year-old that proves they are not 100 percent effective.”
Our next post investigates a little known obstacle to ending mass incarceration in America. To get notified when we post it → join our email list.
This post was written by Rosie Cima. You can follow her on Twitter here.



